At the beginning let’s figure out what this standard is and why it is essential to electronic medical devices. IEC 60601-1 provides general requirements in a series of standards for electronic medical devices that specifically relate to patient safety. In this blog, you will have a better understanding of what IEC 60601-1 means, why it is important, and what is the benefit to get this certification.
What Is IEC 60601-1?
The International Electrotechnical Commission (IEC) is the world’s leading organization that publishes International Standards for all electrical related technologies. Such as IEC 60601-1, which focuses on the safety of electronic medical devices.
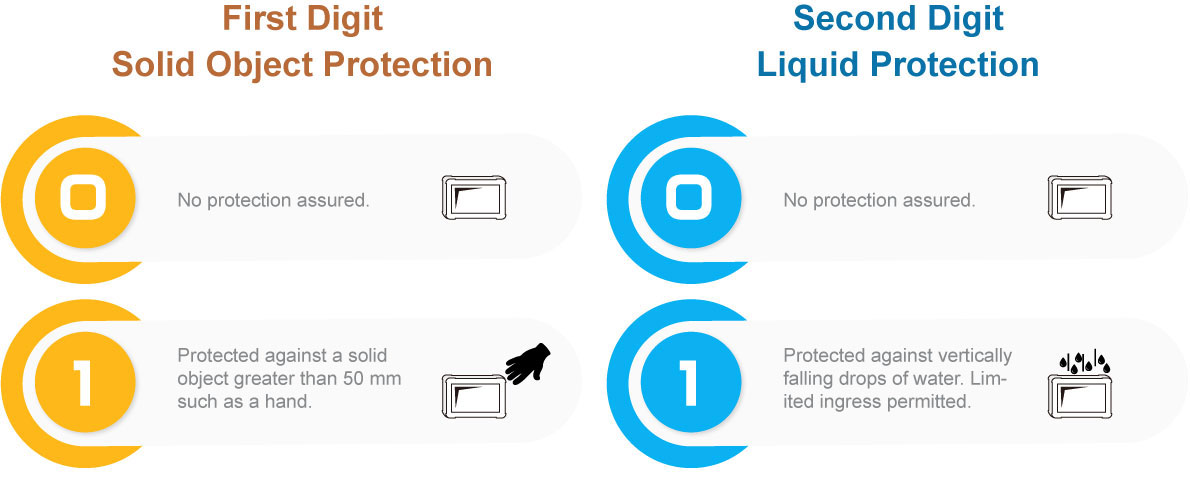
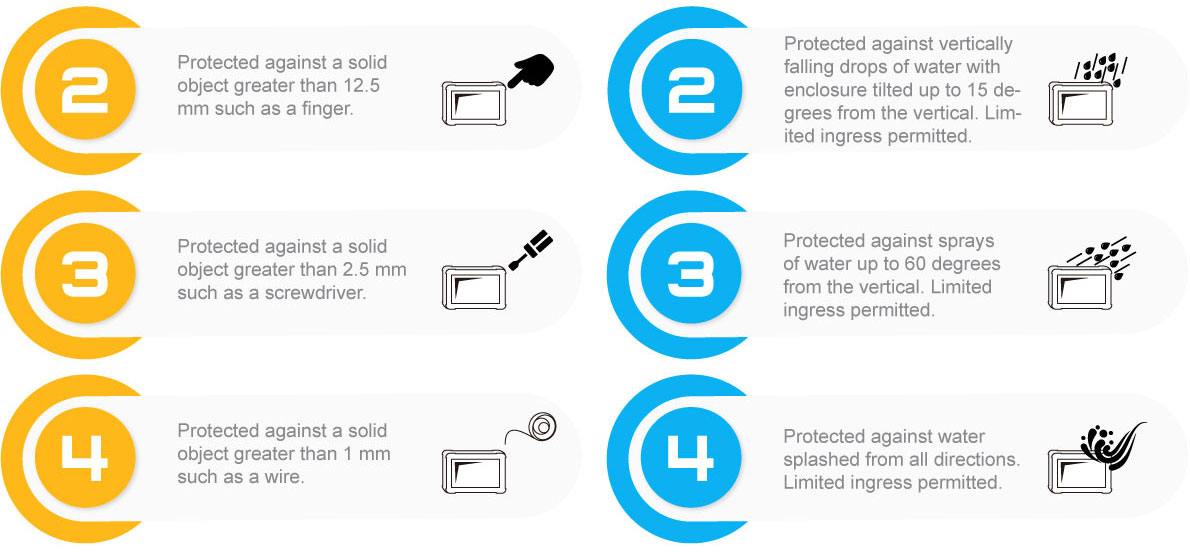
The IEC 60601-1 gets stricter today as an improvement against the rising number of wireless devices in healthcare facilities. It is important to protect electronic medical devices and reduce patient risk from electromagnetic interference(EMI) and electrostatic discharge(ESD). Within IEC 60601-1, there are collateral standards; for example, IEC 60601-1-2 is the electromagnetic compatibility(EMC) collateral standard, which requires that basic safety and essential performance of the electronic medical device be maintained in the presence of electromagnetic disturbances.
What Is New About The IEC 60601-1 4th Edition?
The latest IEC 60601-1 4th edition mainly focuses on EMC issues covered by the collateral standard IEC 60601-1-2. The IEC 60601-1-2 has been developed in response to the rise of more complex wireless devices within hospitals, which include high-frequency WiFi, Bluetooth and smartphone signals.
The IEC 60601-1-2 4th edition has been revised to make sure that all electronic medical devices comply with new requirements for electromagnetic compatibility and immunity. And the most notable changes in the newest 4th edition are the increased electrostatic discharge level; the air discharge requirement raised from 8KV to 15KV and contact discharge requirement has also been increased from 6KV up to 8KV.
Why It Is Important?
We can all agree that when it comes to the benefits we get from medical technology. However, potential risks come out as with all the progress. Today's medical environment is becoming more and more digital and you can see many electronic medical devices be applied in this field. But that also exposes patients to potential risks such as electromagnetic interference generated in various devices.
With an IEC 60601-1-2 4th edition certified electronic medical device, you can make sure a safer medical environment and keep the patient from the life-threatening risks.
Are You Ready For IEC 60601-1-2 4th Edition?
It is mandatory to be compliance with IEC 60601-1-2 4th edition since January 1, 2019 for all electronic medical devices going into the United States, Europe, and Canada. Although this does not cover wireless-enabled IT devices such as computers and tablets, we still recommend that all electronic medical devices should comply with the requirement of IEC 60601-1-2 4th edition to make sure the safety of patients.
How And Where Does A Medical Tablet Be Used?
There are so many practical applications of medical tablets in healthcare. And they have been widely adopted due to the increased awareness of hardware security to protect personal privacy and the efficiency it brings to the medical field.
MACTRON GROUP(MTG) Medical Tablet MMS Series with IEC 60601-1-2 4th edition ensures a long life-cycle for healthcare use and it is used in a lot of medical settings such as remote healthcare and patient registration.
Hospital staff can easily record patient medical information in real-time with WiFi or LTE connectivity, cutting down double-entry time and human errors. Besides, it helps you to eliminate all paperwork and its Anti-Bacteria coating can prevent the spread of germs, unlike clipboards that are passed from one sick patient to the next.
For more information about how MTG’s certified Medical Tablet
MMS Series can fit into your next project, you can
Contact us here.